Our Research
proteostasis / ˈprō-ˌ tē-ō-ˈstā-səs / n. a portmanteau of the words protein and homeostasis. The concept that there are competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking and degradation of proteins present within and outside the cell1,2.
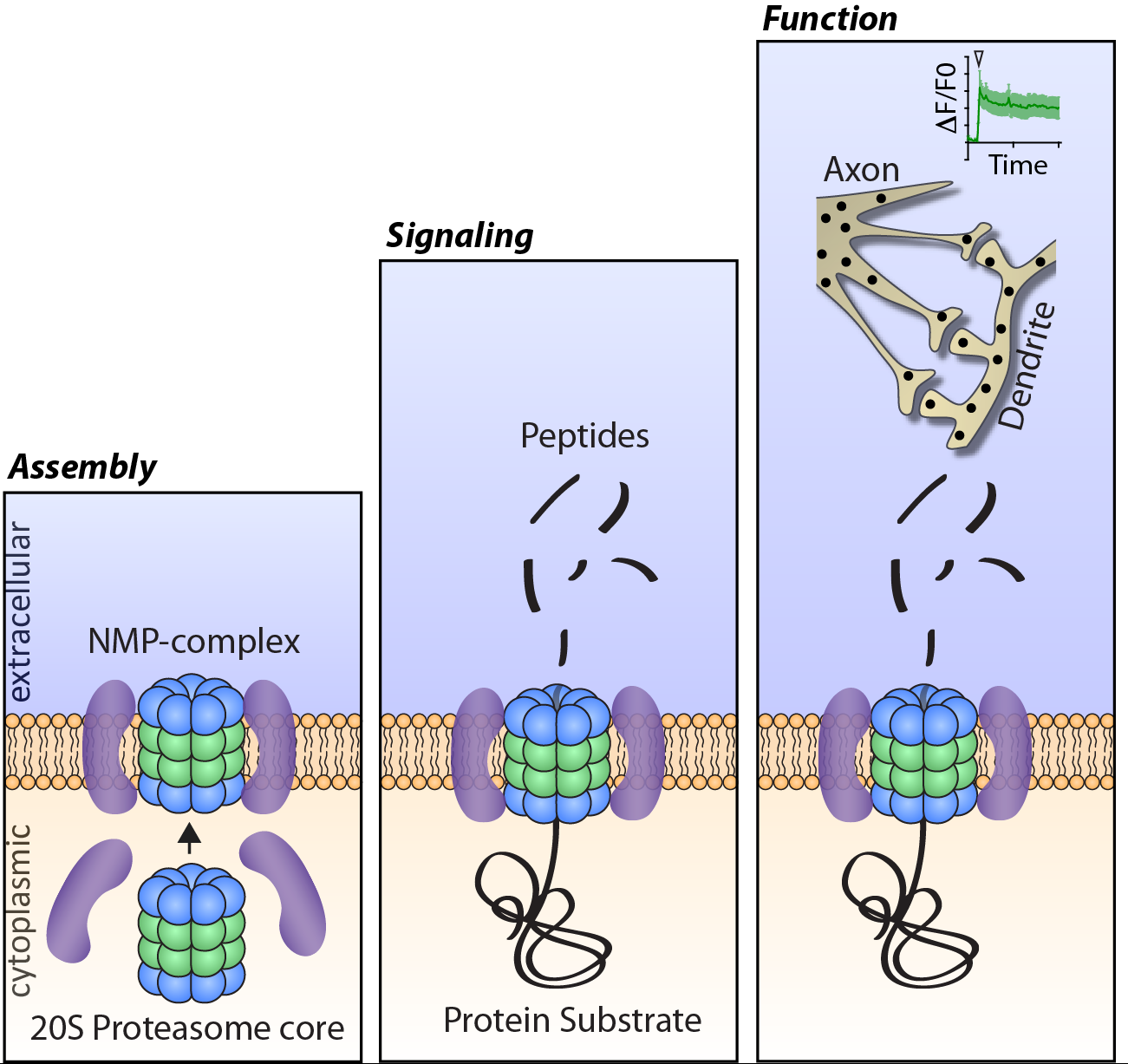
Our laboratory aims to understand how proteostasis machinery in neurons control activity-dependent and stress induced changes in the function, physiology and health of the nervous system. Using mammalian primary neuronal cultures and brain tissue we combine multi-disciplinary approaches that include biochemistry, proteomics, molecular biology, cellular biology and neuronal calcium imaging. Through our efforts we have now discovered a novel nervous-system specific membrane associated proteasome complex that degrades intracellular protein into extracellular peptides with the capacity to potently and specifically modulate neuronal signaling (Ramachandran KV and Margolis, Nat Struct Mol Biol 2017). This discovery of the neuronal membrane proteasome complex (NMP) has revealed a new signaling paradigm and led to the identification of a unique class of neuronal signaling molecules in the form of peptides within the nervous system. Current efforts are being made to understand the molecular mechanisms that control the assembly of this complex, the intracellular source of these peptides and how peptides mediate specific signaling within the nervous system relevant to brain function in health and disease.
synaptopathy / ˈsi-ˌnap-,ˈtä-pə-thē / n. a portmanteau of the words synapse and neuropathy, describing disease of the brain, spinal cord or peripheral nervous system relating to the dysfunction of synapses.
In addition, our laboratory aims to understand the underlying cause of synaptopathies in the human population. We use genetically engineered mouse models of disease and a multidisciplinary approach that combines aspect of biochemistry, in vivo imaging of dendritic spines (location of >95% of all excitatory synapses), electrophysiology and animal behavior. Combining these approaches we investigate and test hypothesis about the underlying cause of synapse degeneration and cognitive decline. Toward this end we have spent time studying endogenous inhibitors of dendritic spine and excitatory synapses formation and function that are deactivated during normal neuronal development. Through our efforts we have learned that these inhibitors are reactivated in human brain disease and mouse models of brain disease. Thus, we have applied considerable focus on testing our central hypothesis that the reactivation of endogenous inhibitors of synapses is an underlying cause of neurodevelopmental and neurodegenerative disease. We then use genetic rescue or stereotactic surgical approaches to target endogenous inhibitors of synapses followed by a wide range of behavioral tasks to assay cognitive function as a proof-of-concept treatment approach. Such efforts are illustrated in a recent study identifying a significant role for the synapse inhibitor Ephexin5 in the development of Alzheimer’s disease (Sell, GL et al., J Clin Invest 2017). Similar studies in our laboratory are looking at another disorder Angelman syndrome. Efforts to take global unbiased approaches to find additional reactivated inhibitors of human brain disease are underway. Mechanistic studies in our laboratory have indicated that the reactivation of these programs in part result from defects in proteostasis mechanisms.
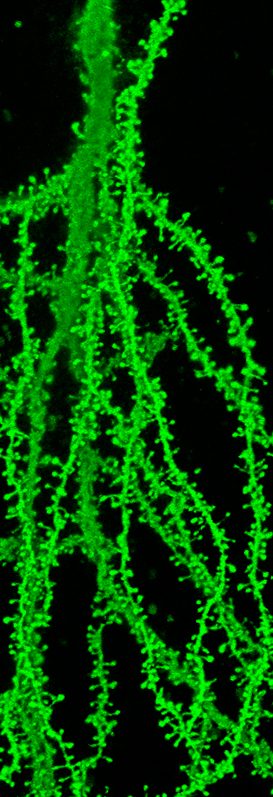
C57BL/6J-Tg(Thy-EGFP)
Postnatal day 30, CA1 hippocampus
- Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916-919, doi:10.1126/science.1141448 (2008).
- Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W. & Balch, W. E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78, 959-991, doi:10.1146/annurev.biochem.052308.114844 (2009).
